Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial - The Lancet
Por um escritor misterioso
Descrição
ESC 2022 – factor XI inhibitors crash

A Multicenter, Phase 2, Randomized, Placebo-Controlled, Double

Pharmacology and Clinical Development of Factor XI Inhibitors

Design and Preclinical Characterization Program toward Asundexian
(BAY 2433334) is an orally active coagulation factor Xia (FXIa) inhibitor. Asundexian binds directly, potently, and reversibly to the active site
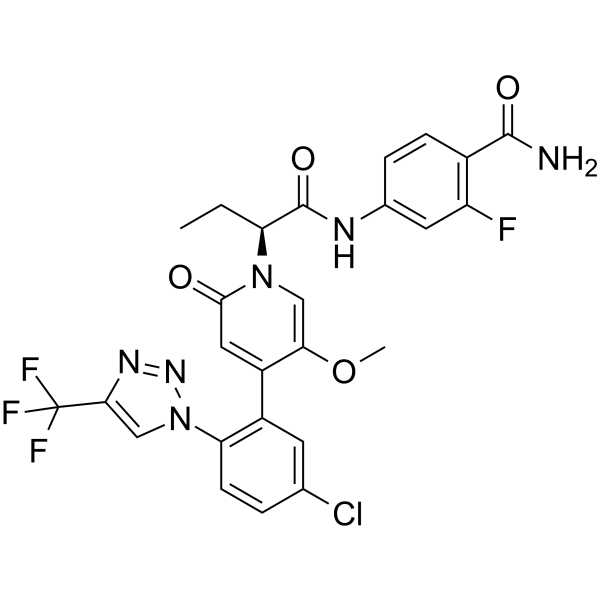
Asundexian
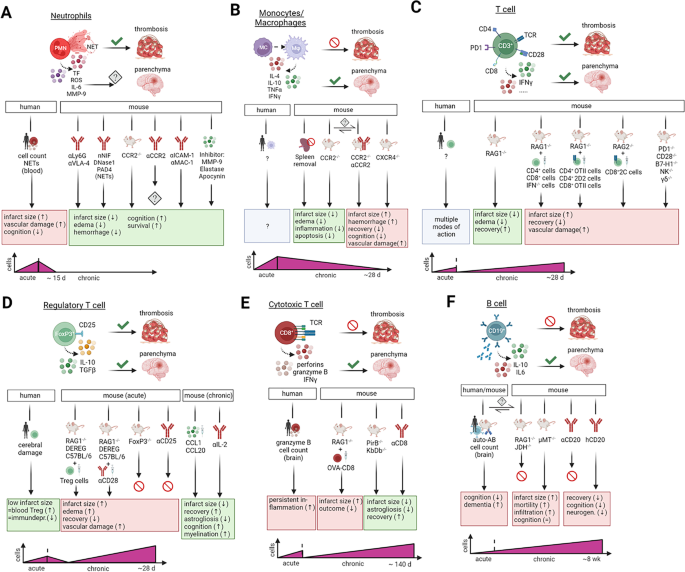
Thromboinflammatory challenges in stroke pathophysiology

ESC 22: Dosing of Asundexian in Pts With Non-Cardioembolic
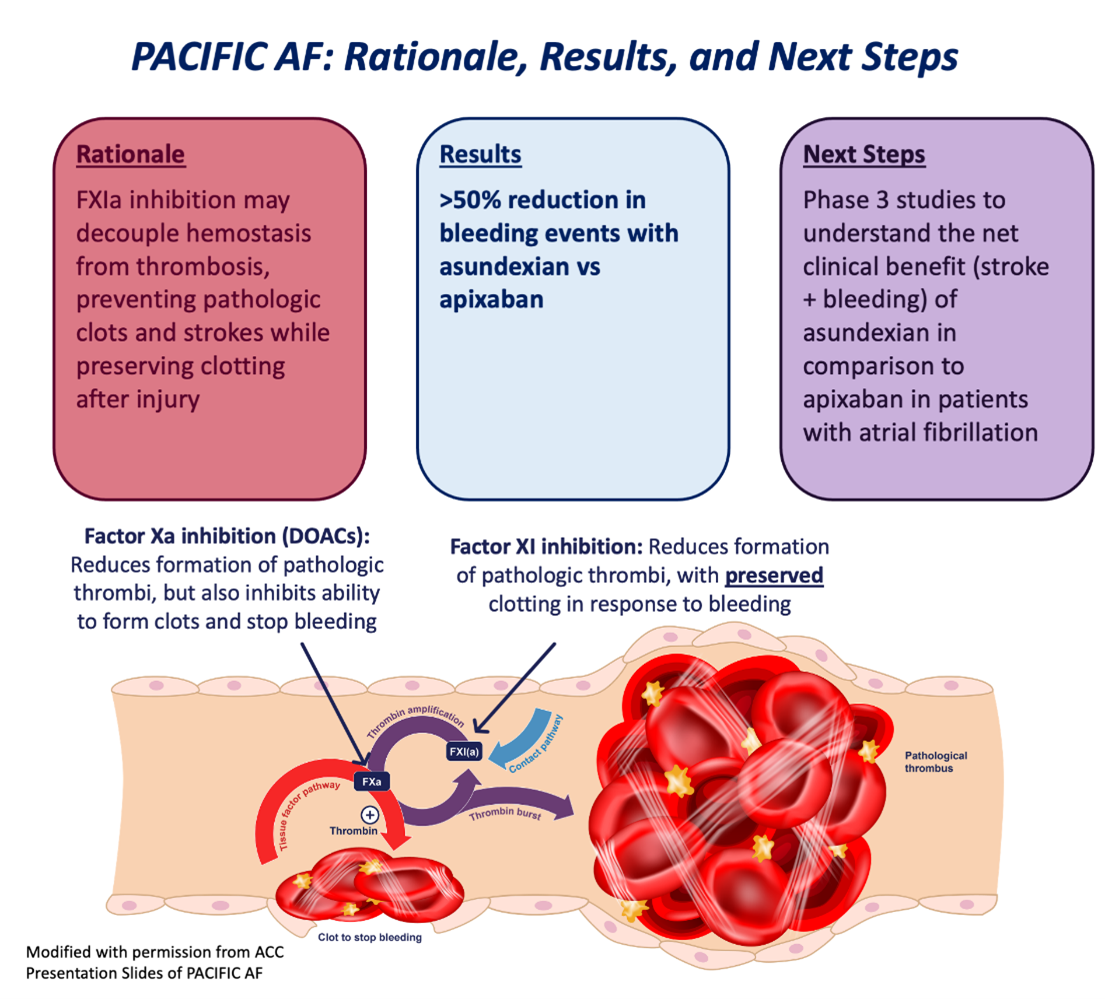
The Next Wave of Anticoagulation: Results of PACIFIC-AF and the

News at XI: moving beyond factor Xa inhibitors - ScienceDirect