FDA grants expanded approval for Bristol Myers Squibb anemia therapy - NJBIZ
Por um escritor misterioso
Descrição
“The approval of Reblozyl in the first-line treatment of anemia for patients with lower-risk MDS represents a crucial step in making transfusion independence possible for more patients," said Tracey Iraca, executive director of the MDS Foundation.

FDA OKs Bristol-Myers Squibb's Breyanzi To Treat Large-B-Cell Lymphoma

Bristol Myers Squibb - U.S. Food and Drug Administration Approves First LAG-3-Blocking Antibody Combination, Opdualag™ (nivolumab and relatlimab-rmbw), as Treatment for Patients with Unresectable or Metastatic Melanoma
/cloudfront-us-east-2.images.arcpublishing.com/reuters/QLJ3TOQERVLHPDYLXAAXK7LAYI.jpg)
U.S. FDA approves new Bristol Myers cancer immunotherapy

Murphy taps NJ civil rights head for high court - NJBIZ

Playing catch-up - NJBIZ

List of top City of New York Venture Stage Investors - Crunchbase Hub Profile

FDA grants breakthrough designation to progressive pulmonary fibrosis treatment

Bristol Myers Squibb - Recent News & Activity
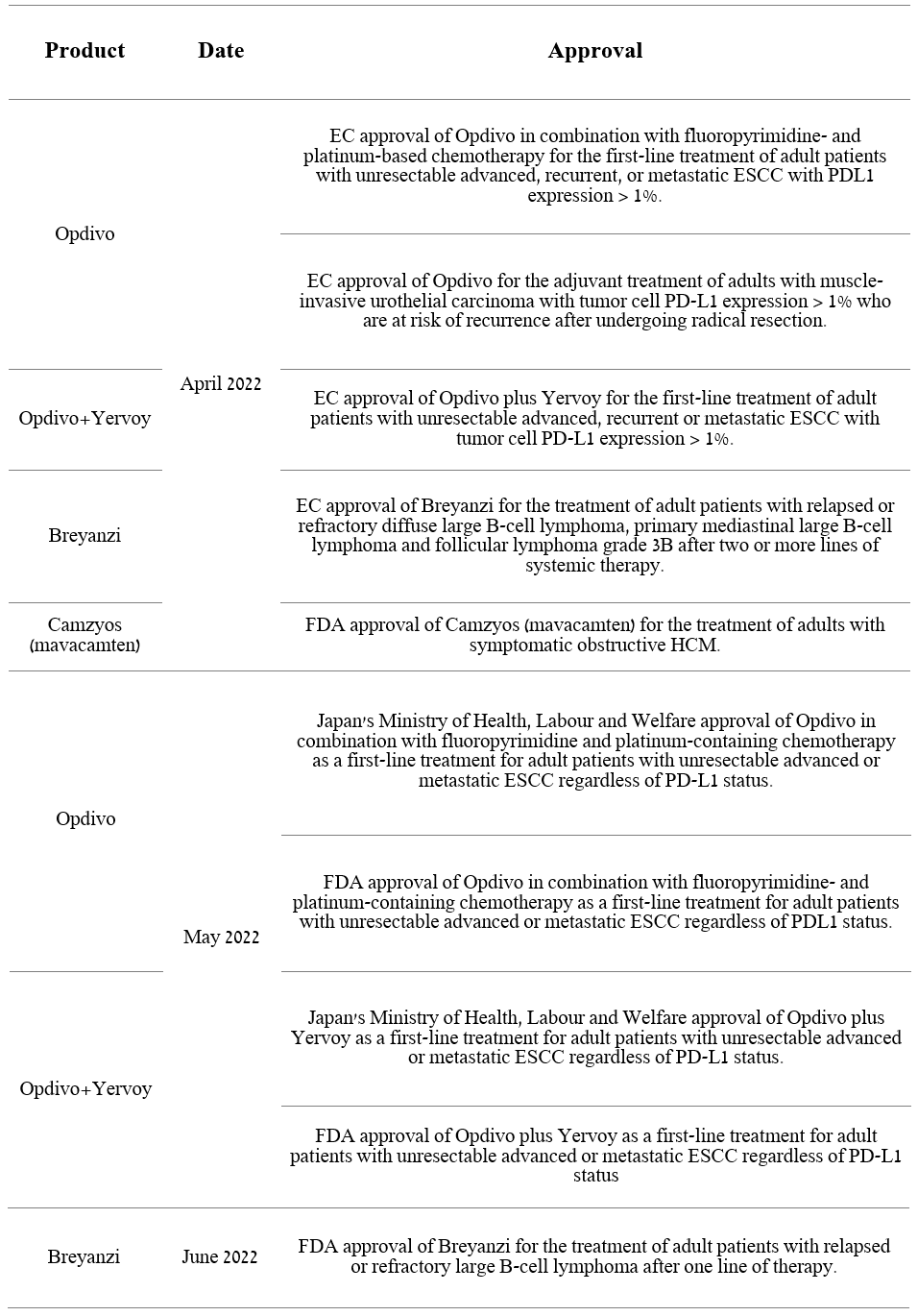
Bristol-Myers Squibb: A Gem Amid Macroeconomic Uncertainties

Four NJ entities to lead new pediatric payment model with $15.8M CMS award - NJBIZ