The Chemokine CCL2 Increases Nav1.8 Sodium Channel Activity in Primary Sensory Neurons through a Gβγ-Dependent Mechanism
Por um escritor misterioso
Descrição
Changes in function of voltage-gated sodium channels in nociceptive primary sensory neurons participate in the development of peripheral hyperexcitability that occurs in neuropathic and inflammatory chronic pain conditions. Among them, the tetrodotoxin-resistant (TTX-R) sodium channel Nav1.8, primarily expressed by small- and medium-sized dorsal root ganglion (DRG) neurons, substantially contributes to the upstroke of action potential in these neurons. Compelling evidence also revealed that the chemokine CCL2 plays a critical role in chronic pain facilitation via its binding to CCR2 receptors. In this study, we therefore investigated the effects of CCL2 on the density and kinetic properties of TTX-R Nav1.8 currents in acutely small/medium dissociated lumbar DRG neurons from naive adult rats. Whole-cell patch-clamp recordings demonstrated that CCL2 concentration-dependently increased TTX-resistant Nav1.8 current densities in both small- and medium-diameter sensory neurons. Incubation with CCL2 also shifted the activation and steady-state inactivation curves of Nav1.8 in a hyperpolarizing direction in small sensory neurons. No change in the activation and inactivation kinetics was, however, observed in medium-sized nociceptive neurons. Our electrophysiological recordings also demonstrated that the selective CCR2 antagonist INCB3344 [ N -[2-[[(3 S ,4 S )-1- E 4-(1,3-benzodioxol-5-yl)-4-hydroxycyclohexyl]-4-ethoxy-3-pyrrolidinyl]amino]-2-oxoethyl]-3-(trifluoromethyl)benzamide] blocks the potentiation of Nav1.8 currents by CCL2 in a concentration-dependent manner. Furthermore, the enhancement in Nav1.8 currents was prevented by pretreatment with pertussis toxin (PTX) or gallein (a Gβγ inhibitor), indicating the involvement of Gβγ released from PTX-sensitive Gi/o-proteins in the cross talk between CCR2 and Nav1.8. Together, our data clearly demonstrate that CCL2 may excite primary sensory neurons by acting on the biophysical properties of Nav1.8 currents via a CCR2/Gβγ-dependent mechanism.

PDF) The Chemokine CCL2 Increases Nav1.8 Sodium Channel Activity in Primary Sensory Neurons through a G -Dependent Mechanism

Na v 1.8 currents are increased in small sensory neurons following

Chemokine CCL7 mediates trigeminal neuropathic pain via CCR2/CCR3-ERK pathway in the trigeminal ganglion of mice - Lin-Peng Zhu, Meng-Lin Xu, Bao-Tong Yuan, Ling-Jie Ma, Yong-Jing Gao, 2023

TRPV 1 receptor inhibition decreases CCL 2-induced hyperalgesia
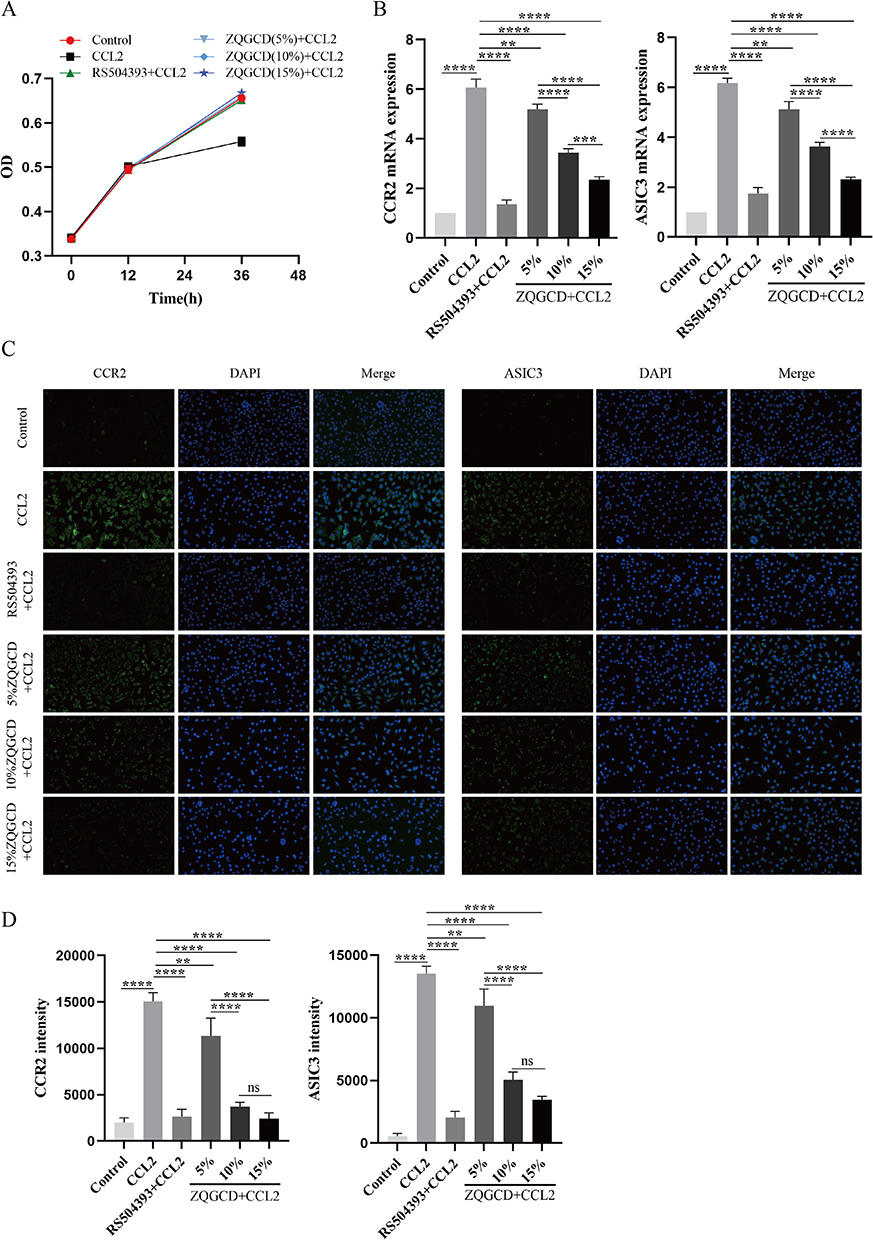
Zhiqiao Gancao decoction on hyperalgesia in LDH

Functional inhibition of chemokine receptor CCR2 by dicer-substrate-siRNA prevents pain development - Valérie Bégin-Lavallée, Élora Midavaine, Marc-André Dansereau, Pascal Tétreault, Jean-Michel Longpré, Ashley M Jacobi, Scott D Rose, Mark A Behlke

Neonatal Injury Modulates Incisional Pain Sensitivity in Adulthood: An Animal Study - ScienceDirect
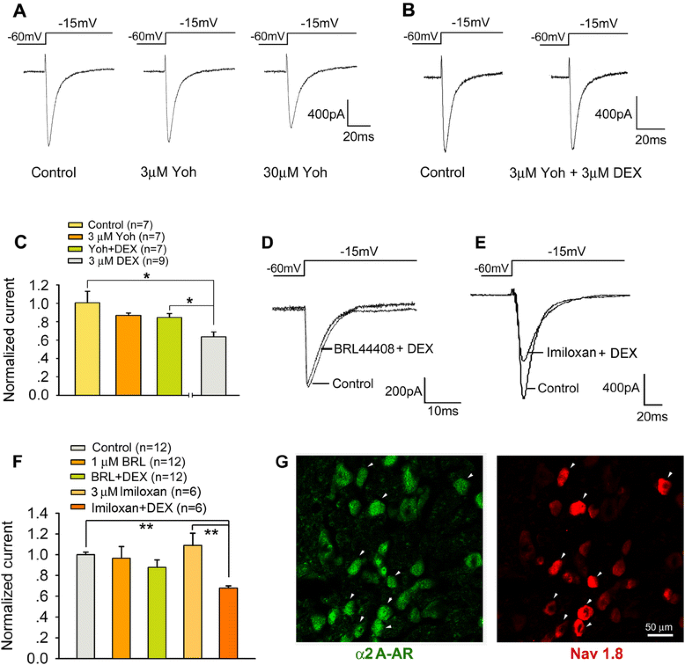
Dexmedetomidine inhibits Tetrodotoxin-resistant Nav1.8 sodium channel activity through Gi/o-dependent pathway in rat dorsal root ganglion neurons, Molecular Brain
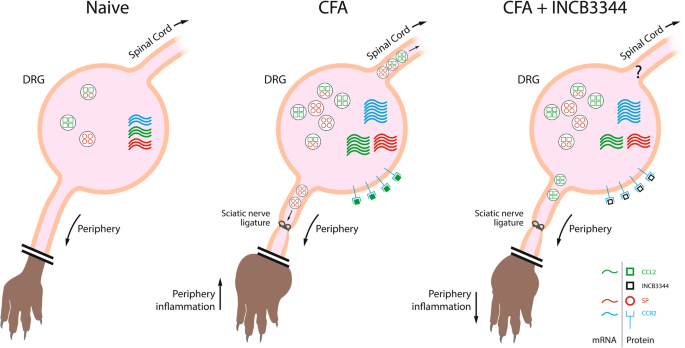
Mechanistic insights into the role of the chemokine CCL2/CCR2 axis in dorsal root ganglia to peripheral inflammation and pain hypersensitivity, Journal of Neuroinflammation

Neonatal Injury Modulates Incisional Pain Sensitivity in Adulthood: An Animal Study - ScienceDirect
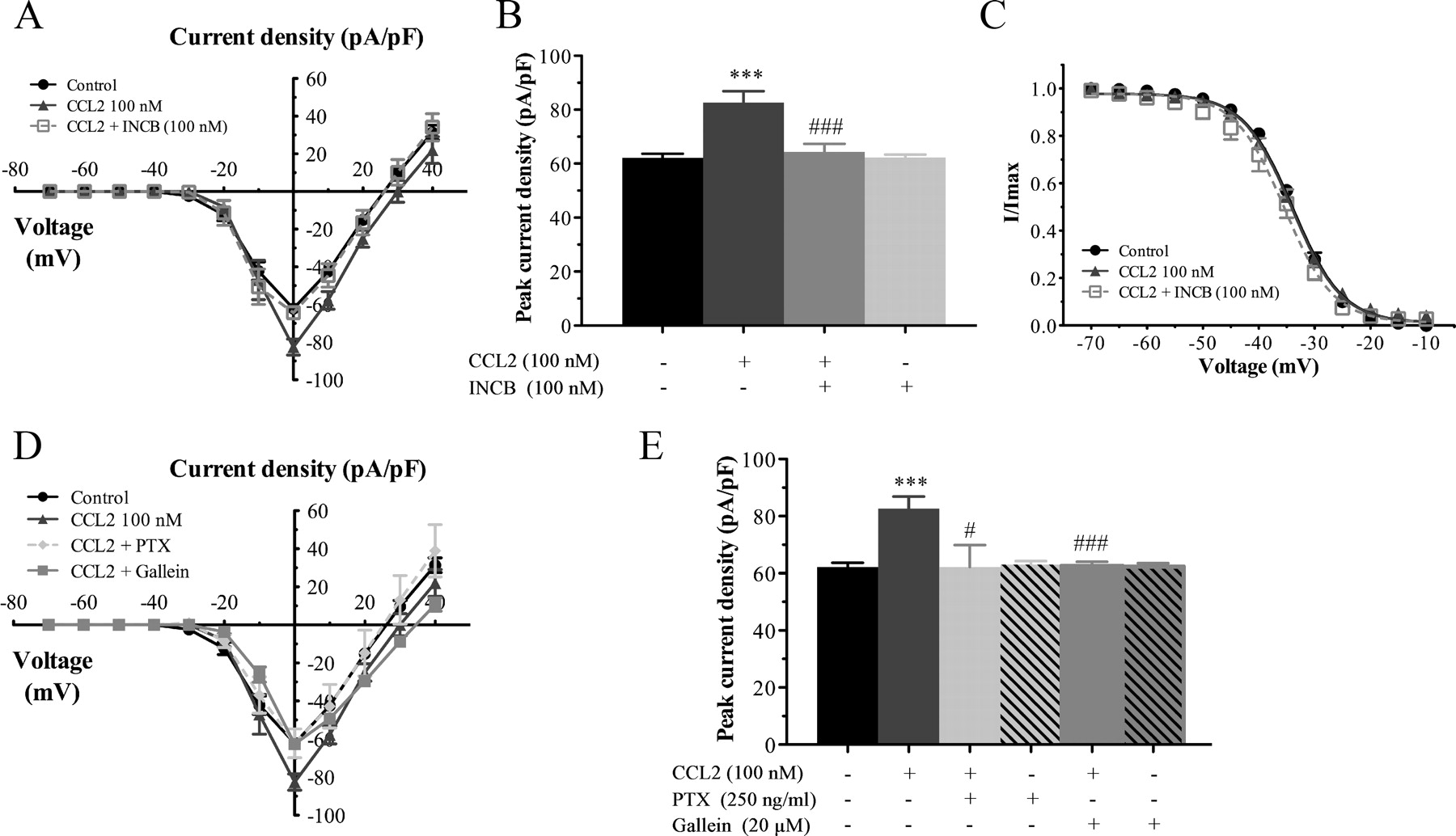
The Chemokine CCL2 Increases Nav1.8 Sodium Channel Activity in Primary Sensory Neurons through a Gβγ-Dependent Mechanism

Chemokine CCL7 mediates trigeminal neuropathic pain via CCR2/CCR3-ERK pathway in the trigeminal ganglion of mice - Lin-Peng Zhu, Meng-Lin Xu, Bao-Tong Yuan, Ling-Jie Ma, Yong-Jing Gao, 2023
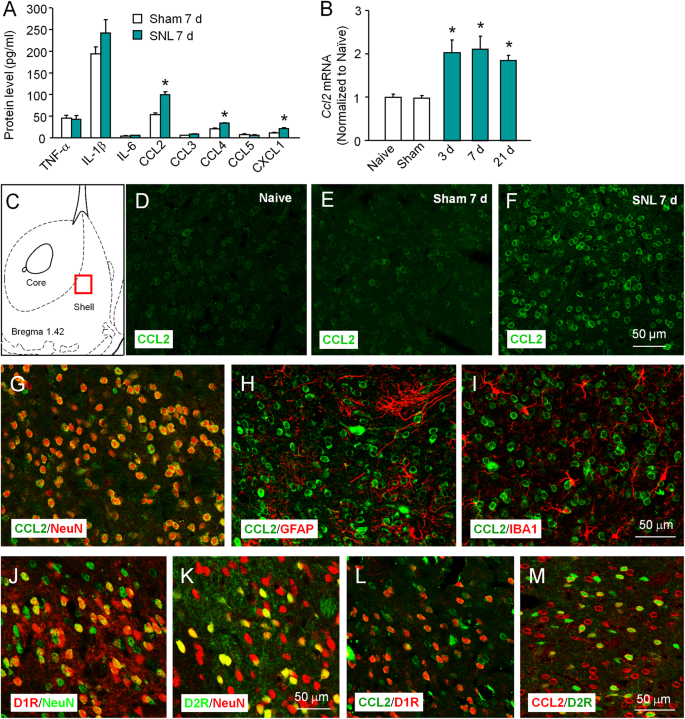
Chemokine receptor CCR2 contributes to neuropathic pain and the associated depression via increasing NR2B-mediated currents in both D1 and D2 dopamine receptor-containing medium spiny neurons in the nucleus accumbens shell

Functional inhibition of chemokine receptor CCR2 by dicer-substrate-siRNA prevents pain development - Valérie Bégin-Lavallée, Élora Midavaine, Marc-André Dansereau, Pascal Tétreault, Jean-Michel Longpré, Ashley M Jacobi, Scott D Rose, Mark A Behlke