Top 4 eConsent Questions in Clinical Research: Forms & More
Por um escritor misterioso
Descrição

5 myths about eConsent
Electronic Data Capture Software: 5 Apps for Clinical Research

Econsent Solutions: A Better Way to Process Informed Consent in

Econsent

Paving the way to a more effective informed consent process
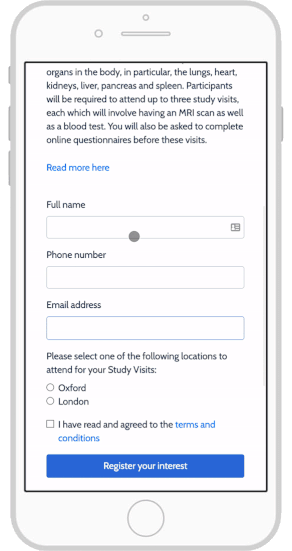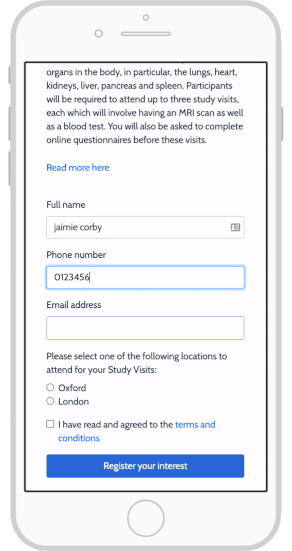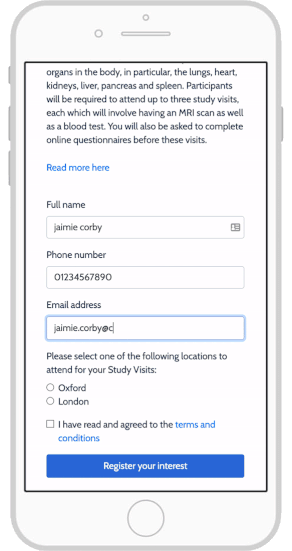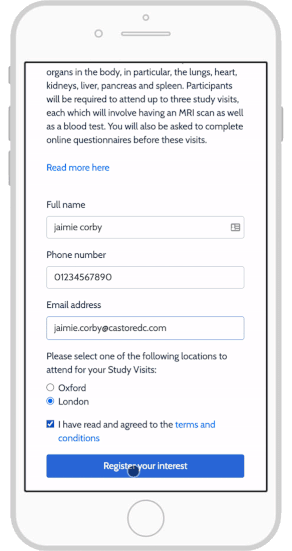
Top 4 eConsent Questions in Clinical Research: Forms & More

EvidentIQ eConsent 2023 Pricing, Features, Reviews & Alternatives
Top 4 eConsent Questions in Clinical Research: Forms & More
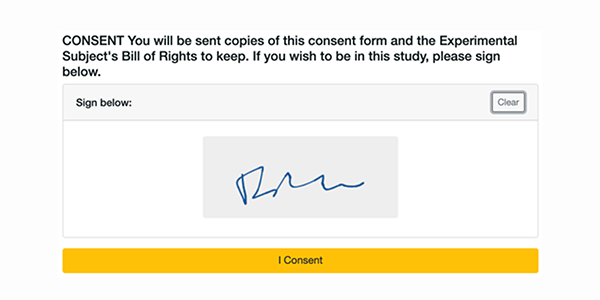
Using e-Consent Forms in Your Clinical Research

E consent for research: Considerations in Implementation and IRB
How to Avoid the Top 5 Clinical Trial FDA Inspection Failures
Remote & Virtual Trials
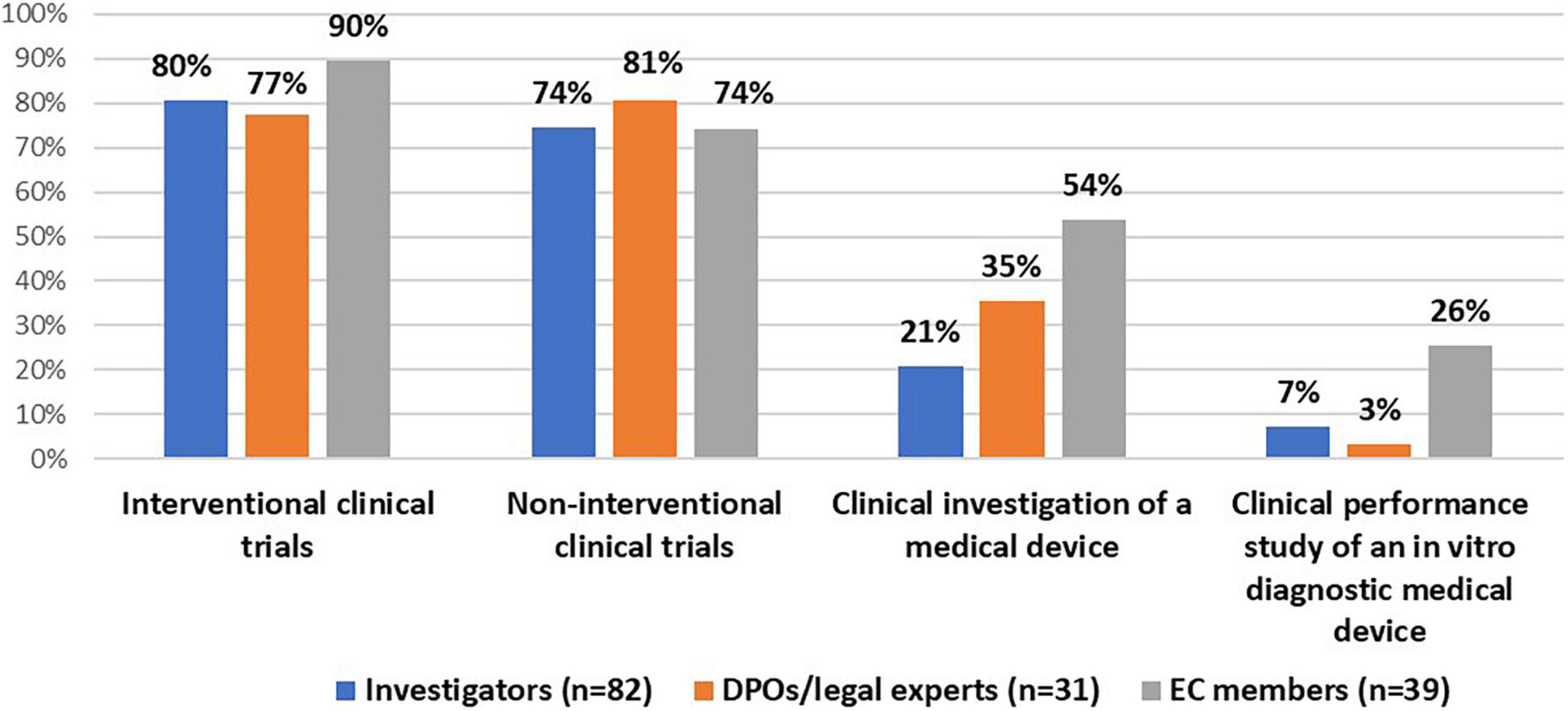
Frontiers Rethinking informed consent in the time of COVID-19