Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV
Por um escritor misterioso
Descrição
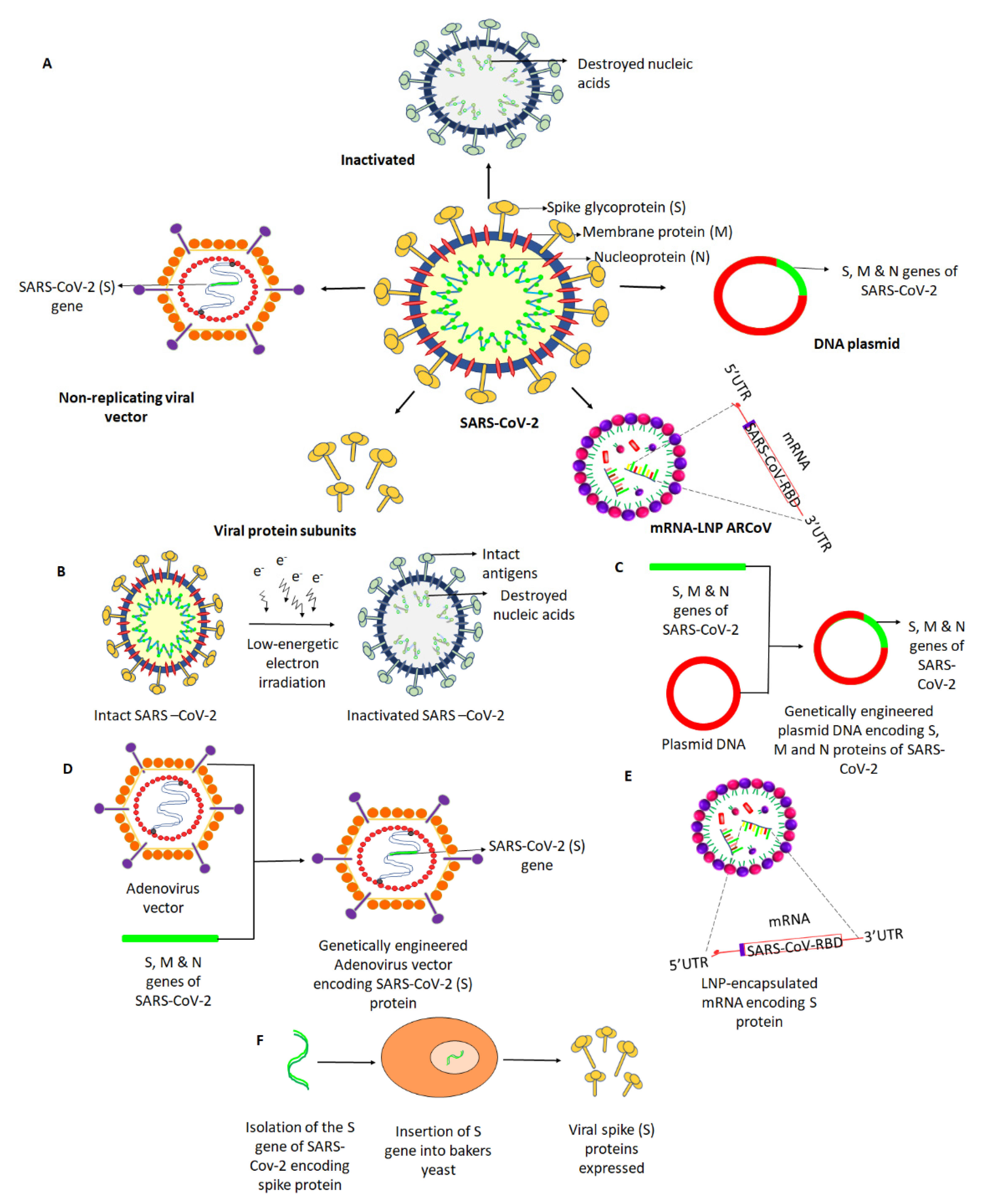
Vaccines, Free Full-Text

COVID-19 Vaccine Frontrunners and Their Nanotechnology Design

Coronavirus vaccine development: from SARS and MERS to COVID-19, Journal of Biomedical Science
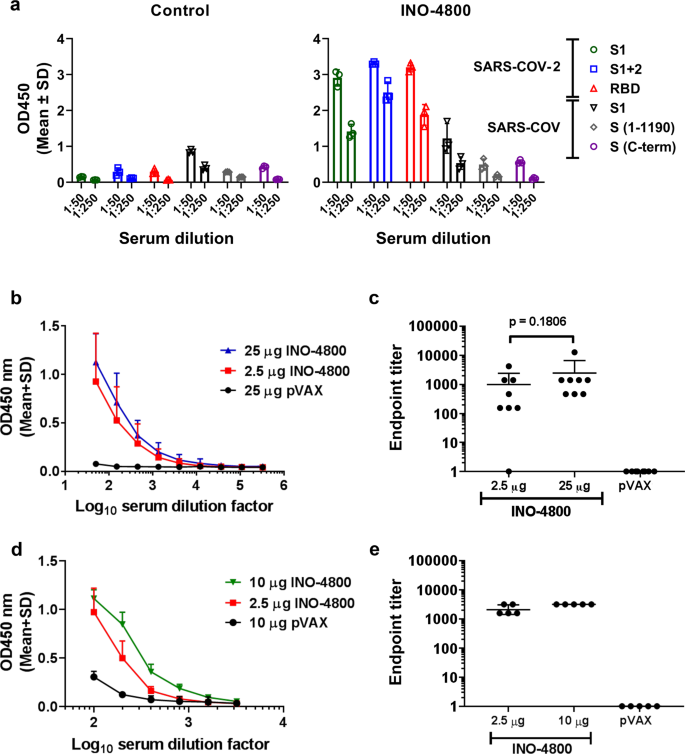
Immunogenicity of a DNA vaccine candidate for COVID-19

COVID-eVax, an electroporated DNA vaccine candidate encoding the SARS-CoV-2 RBD, elicits protective responses in animal models - ScienceDirect
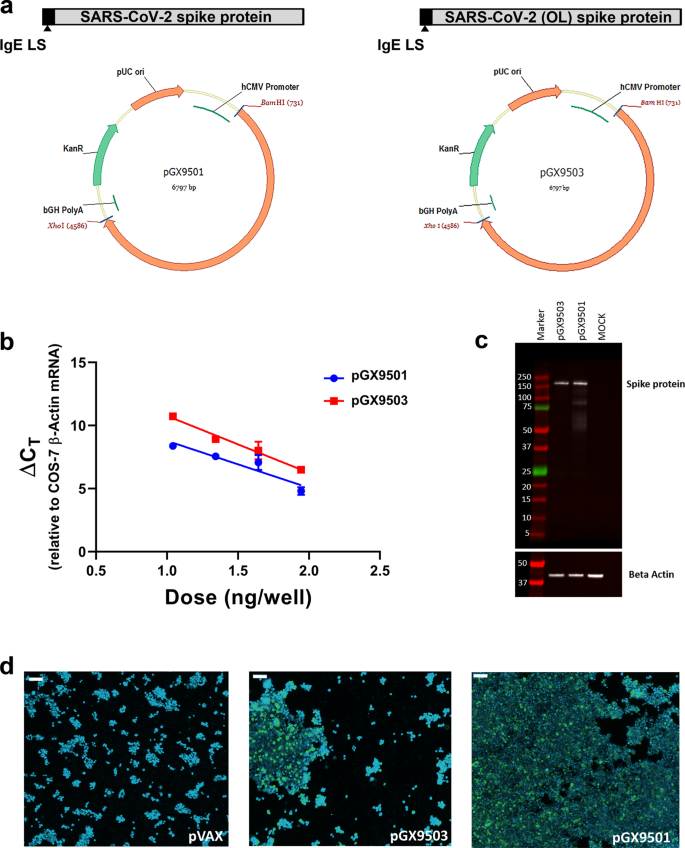
Immunogenicity of a DNA vaccine candidate for COVID-19

A Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development
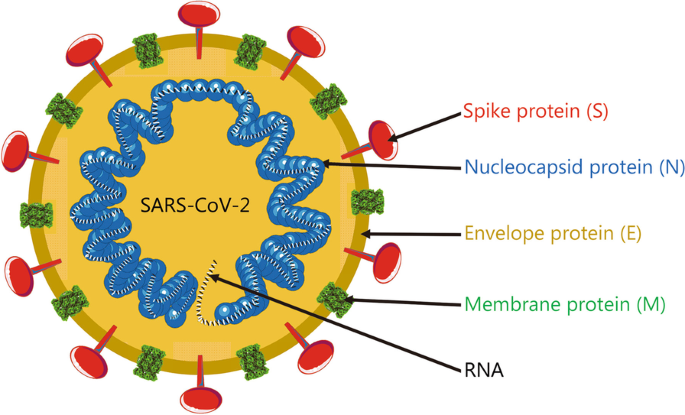
Advances in the design and development of SARS-CoV-2 vaccines, Military Medical Research

Enhanced immunogenicity elicited by a novel DNA vaccine encoding the SARS- CoV-2 S1 protein fused to the optimized flagellin of Salmonella typhimurium in mice

Nanotechnology‐facilitated vaccine development during the coronavirus disease 2019 (COVID‐19) pandemic - Wang - 2022 - Exploration - Wiley Online Library
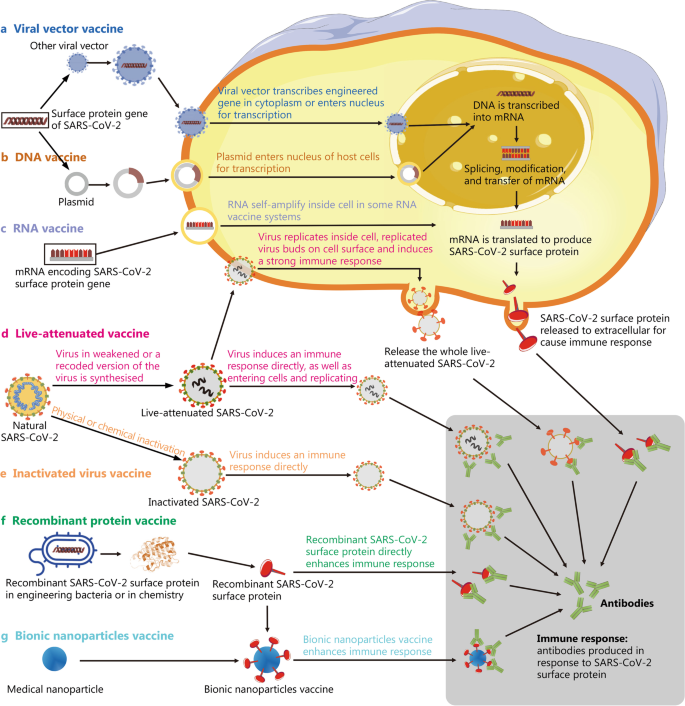
Advances in the design and development of SARS-CoV-2 vaccines, Military Medical Research
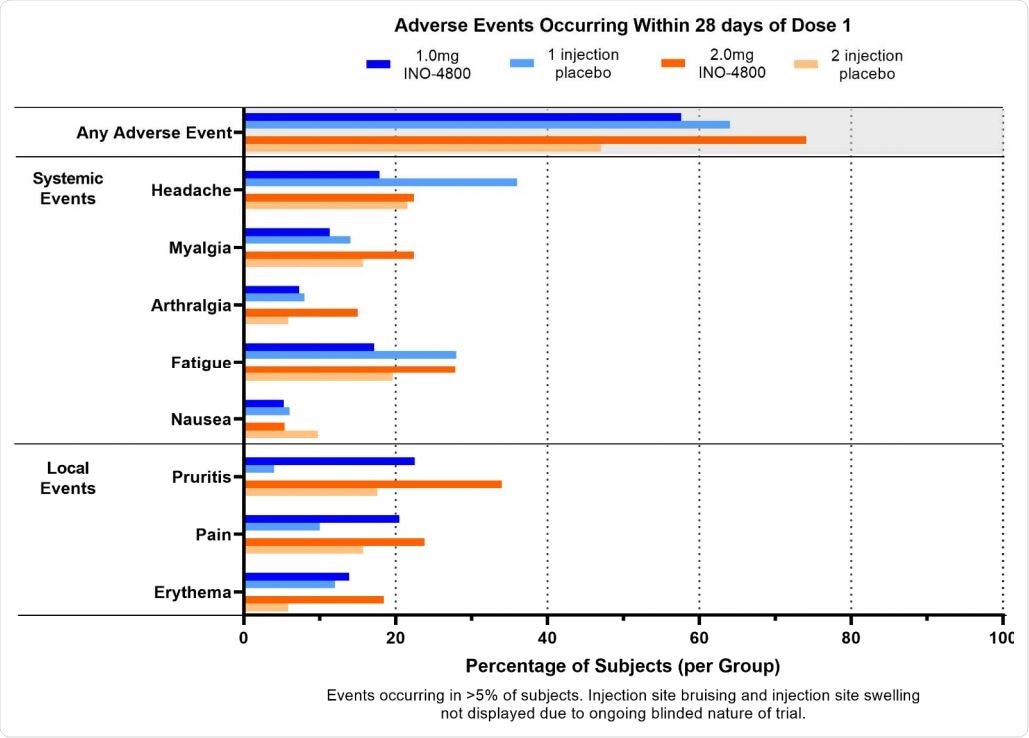
Phase 2 trial shows INO-4800 SARS-CoV-2 DNA vaccine safe and tolerable in adults
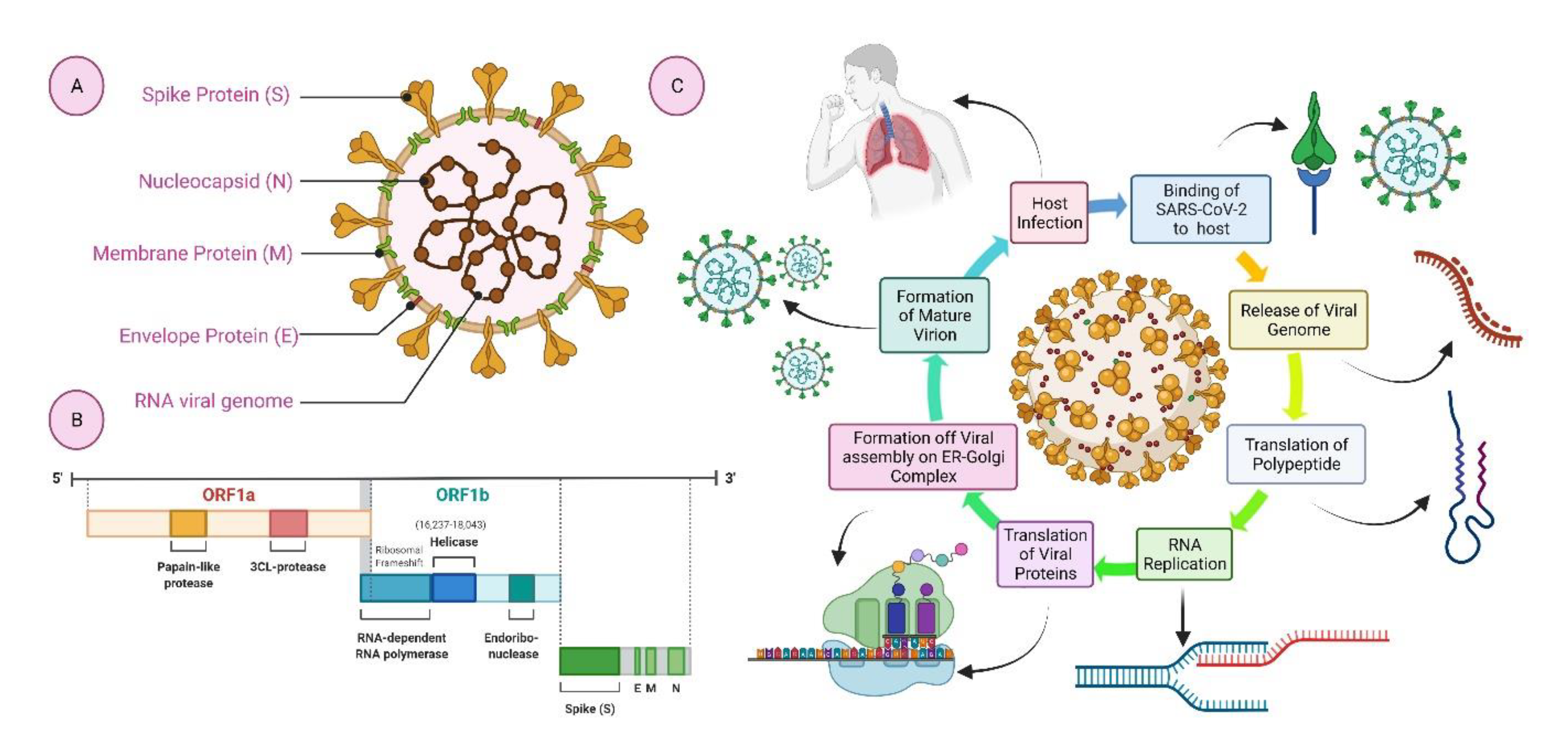
Biologics, Free Full-Text

A comprehensive review on COVID-19 vaccines: development, effectiveness, adverse effects, distribution and challenges
Evaluation of a synthetic DNA SARS-CoV-2 vaccine INO-4800 using a nonhuman primate model



:max_bytes(150000):strip_icc()/banking.asp-Final-e3a67ff9762b40aeac56983c22695032.jpg)



