FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Descrição
Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

Review of 4 cell therapy types under study for COVID-19 - The Niche
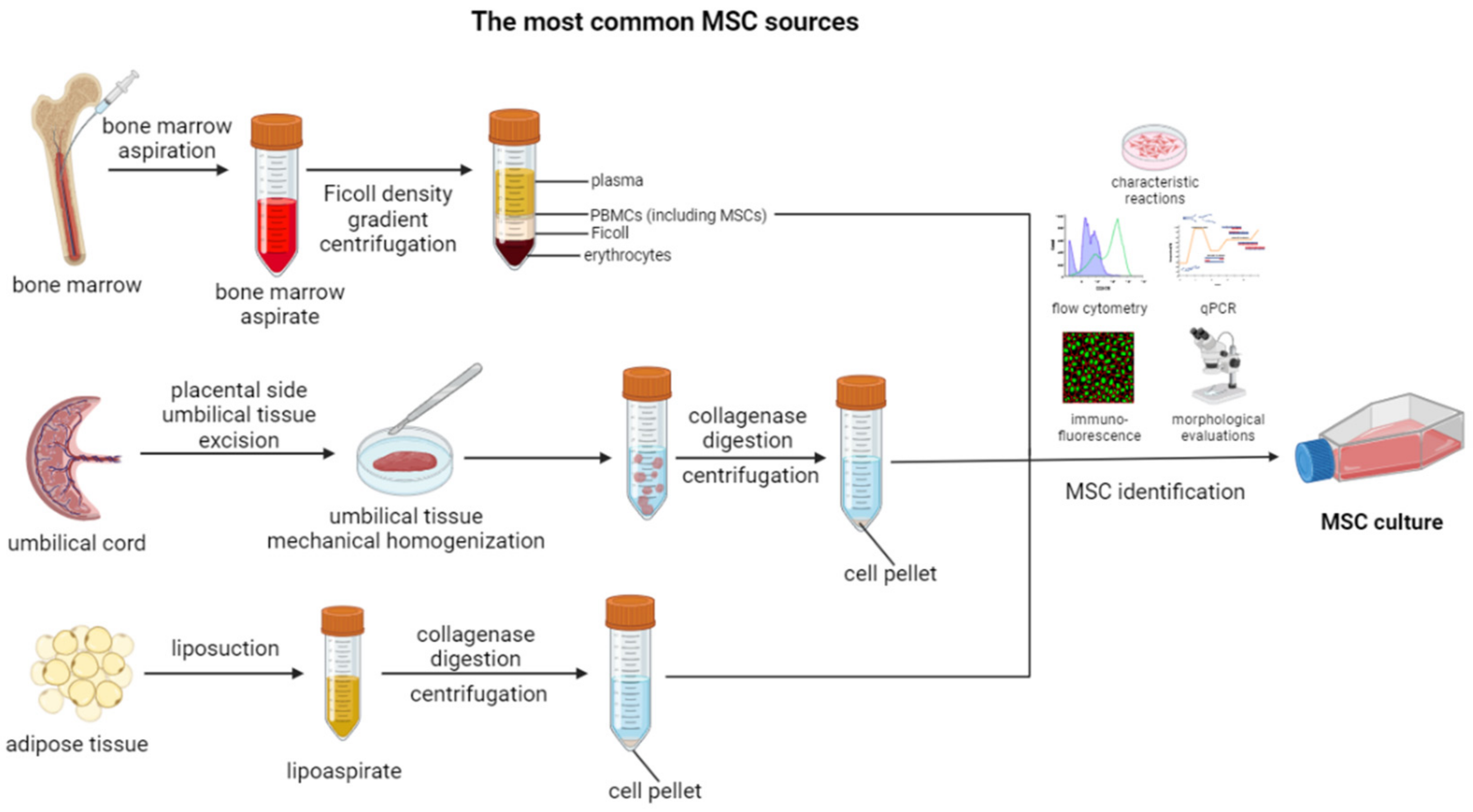
Cells, Free Full-Text

Stem Cell Transplantation, Autologous Stem Cell Transplantation

FDA approves 2 gene therapies for sickle cell disease
:max_bytes(150000):strip_icc()/Health-Stocksy-3418126-80bec79dd1a145419ff8a990f1b32b57.jpg)
FDA Approves Fecal Transplant Therapy for Recurrent C. Diff
/cdn.vox-cdn.com/uploads/chorus_asset/file/13676526/GettyImages_603576872.jpg)
Stem cell therapy: FDA investigates clinics offering unproven

Gene Therapy in Oncology - ScienceDirect

Oligonucleotides to the (Gene) Rescue: FDA Approvals 2017–2019

PDF) Human amniotic mesenchymal stem cells to promote/suppress
T-cell and natural killer cell therapies for hematologic

A Judge Rules Against One Stem-Cell Clinic. There Are Hundreds of







