GHGH Formula - C14H26O11 - Over 100 million chemical compounds
Por um escritor misterioso
Descrição
GHGH contains total 51 atom(s); 26 Hydrogen atom(s), 14 Carbon atom(s), and 11 Oxygen atom(s). Learn more about GHGH chemical formula at Mol-Instincts.
Solved] 14.5 grams of sugar (C12H22O11) are dissolved in water to make 1 L
A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g

degradation of long- chain hydrocarbon compounds

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g
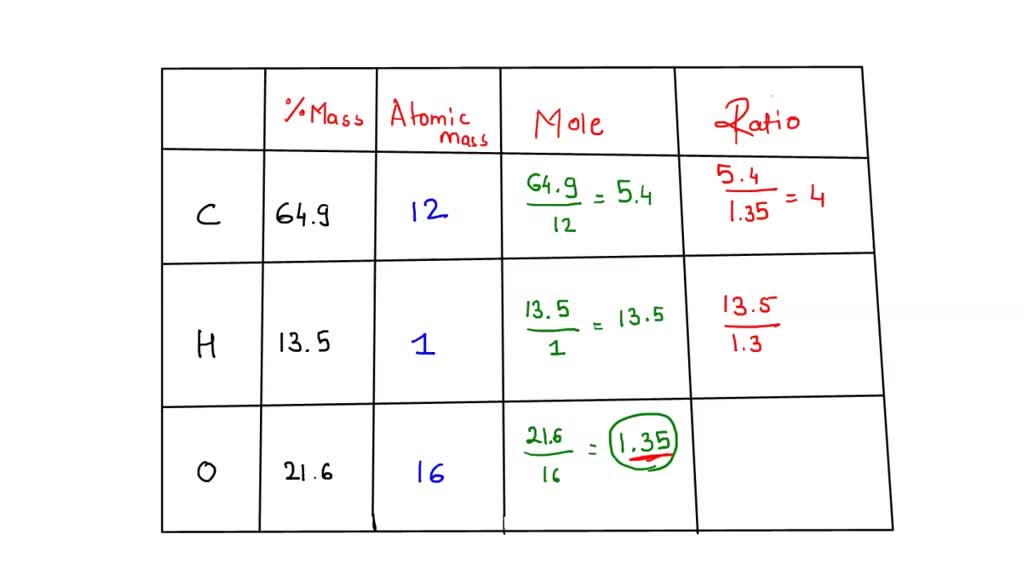
SOLVED: A compound is 64.9% carbon, 13.5% hydrogen, 21.6% oxygen. Its molar mass is 74.0 g/mol. What is its molecular formula?

DeltaDi-HA Molecular Weight - C14H21NO11 - Over 100 million chemical compounds
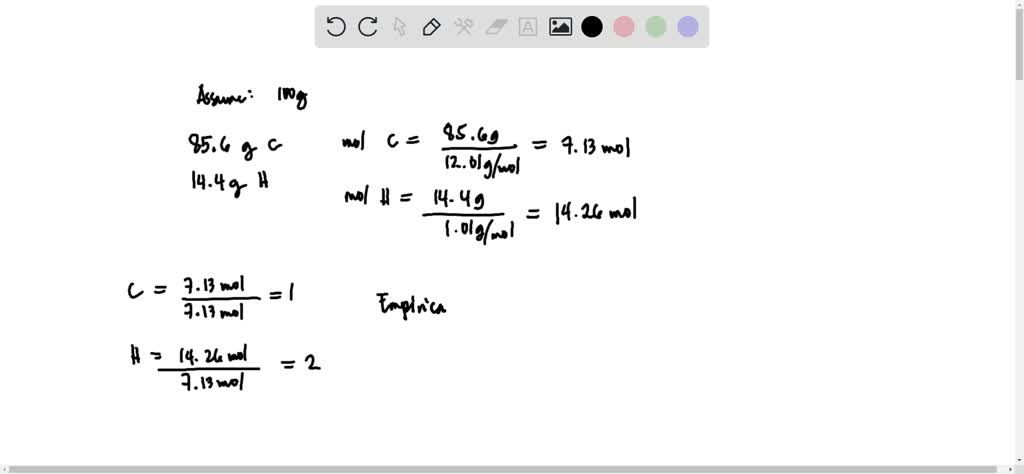
SOLVED: What is the molecular formula of a compound that contains only carbon and hydrogen, is 85.6% carbon, and has a molar mass of 70 g/mol?

Human growth hormone (32-38) Formula - C39H60N8O13 - Over 100 million chemical compounds
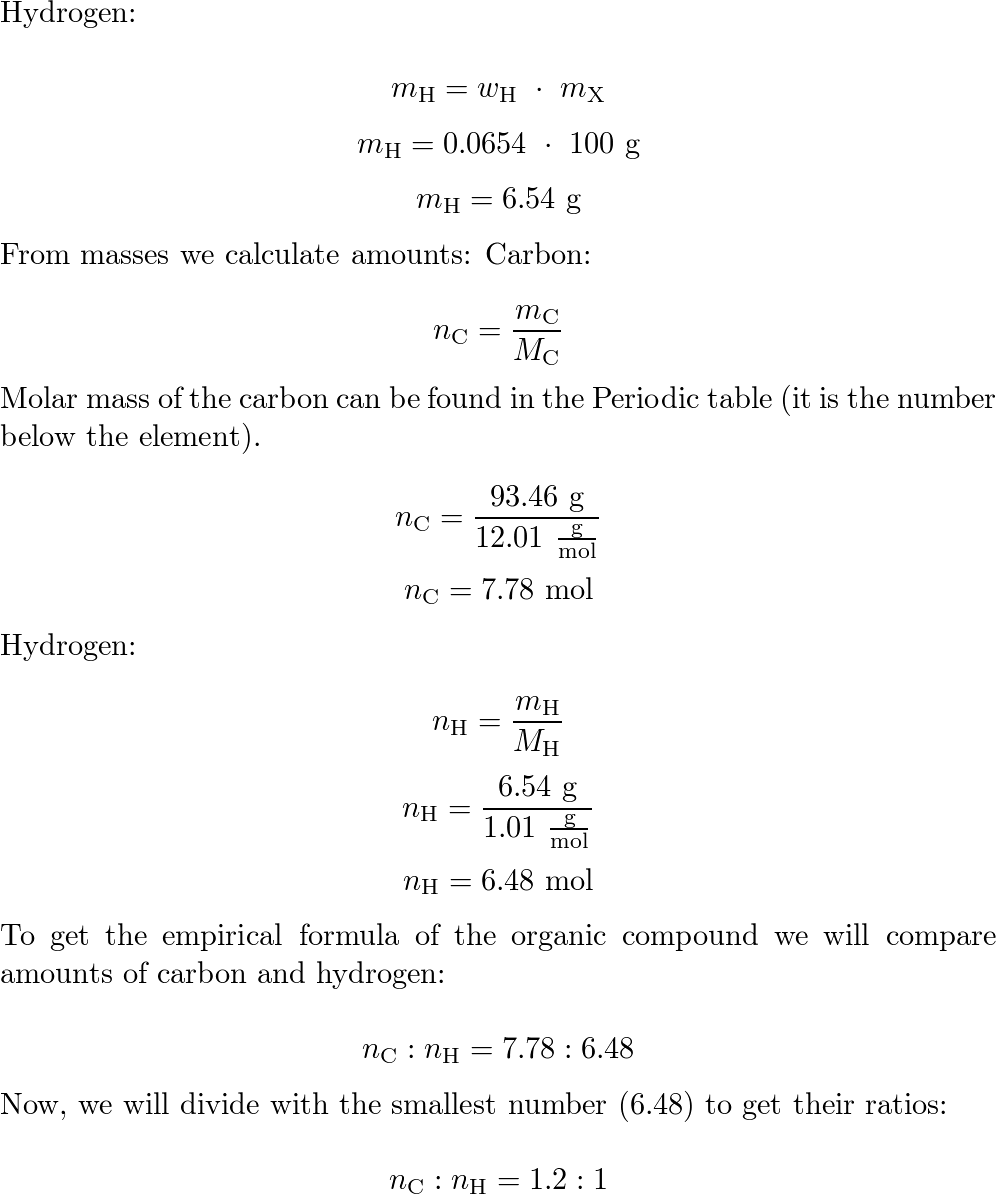
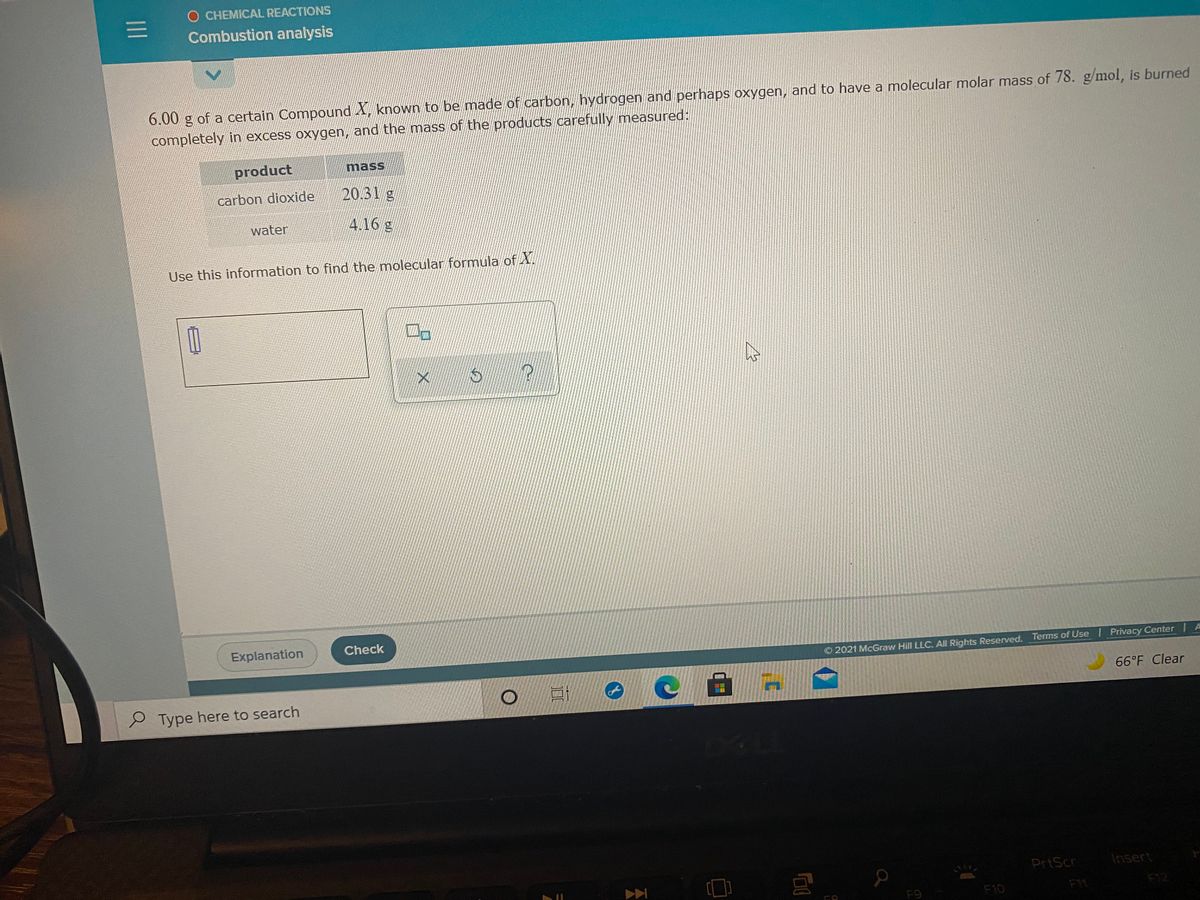
Answered: 6.00 g of a certain Compound X, known…

Anthraquinone contains only carbon, hydrogen, and oxygen. Wh
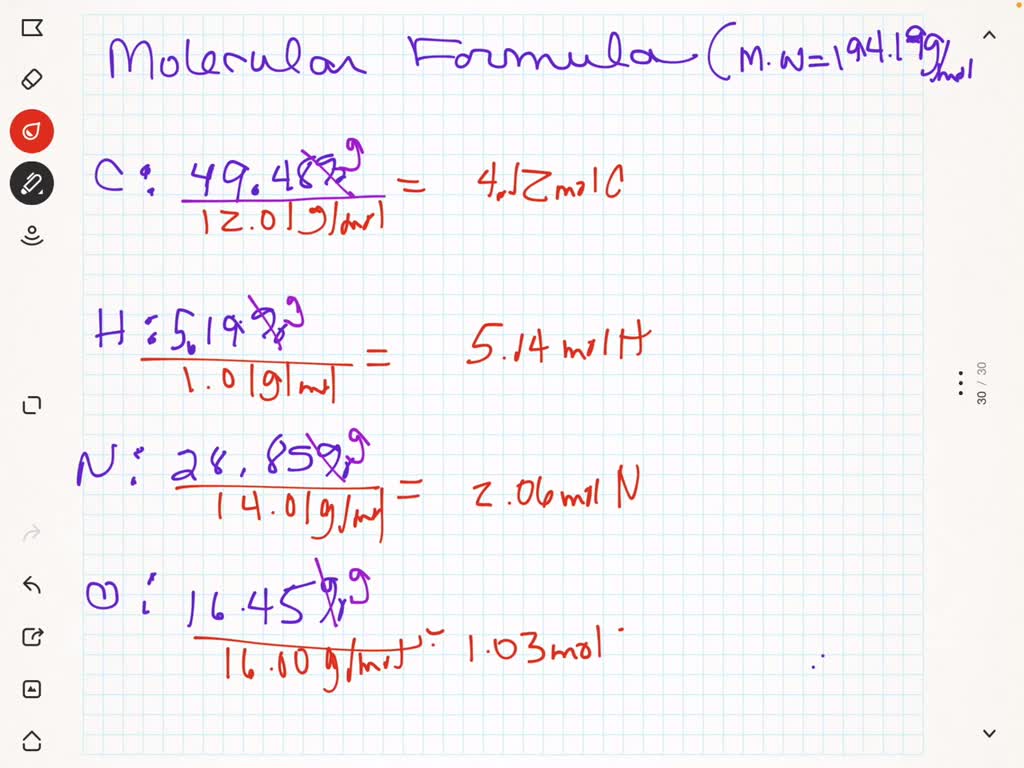
SOLVED: Determine the molecular formula of a compound that is 49.48% carbon, 5.19% hydrogen, 28.85% nitrogen, and 16.48% oxygen. The molecular weight is 194.19 g/mol. C8H12N4O2

0.9 gm of a volatile solid organic compound (molecular weight =90) containing carbon, hydrogen and
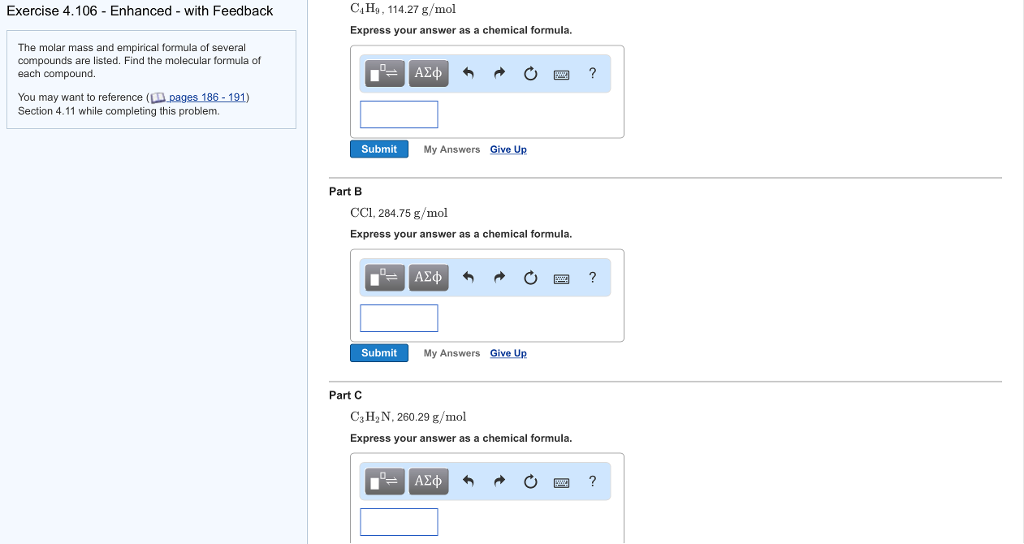
Solved C4Ho, 114.27 g/mol Express your answer as a chemical