Why is Adiabatic Curve steeper than Isothermal Curve
Por um escritor misterioso
Descrição
Compartilhe seus vídeos com amigos, familiares e todo o mundo
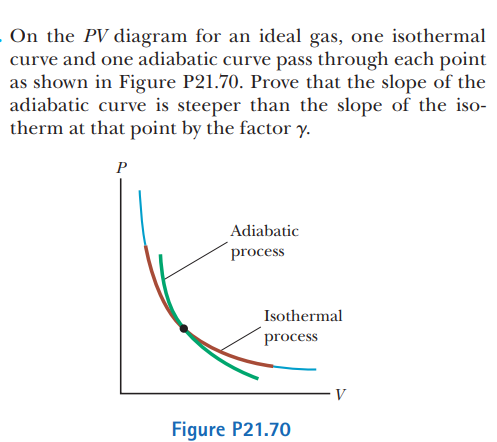
SOLVED: On the P V diagram for an ideal gas, one isothermal curve and one adiabatic curve pass through each point as shown in Figure P21.70. Prove that the slope of the

Adiabatic curve is steeper than the isothermal one explain.
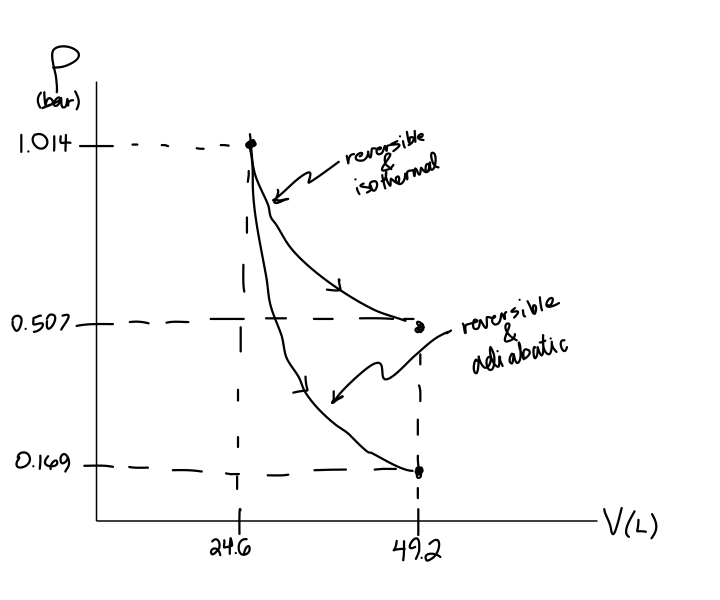
How will you prove that work done in isothermal process is always greater than adiabatic process?

The incorrect figures representing isothermal and adiabatic expansion of an ideal gas from a particular initial state isare:BD
Explain Graphs -- adiabatic Expansion of mono- di- and poly- gas
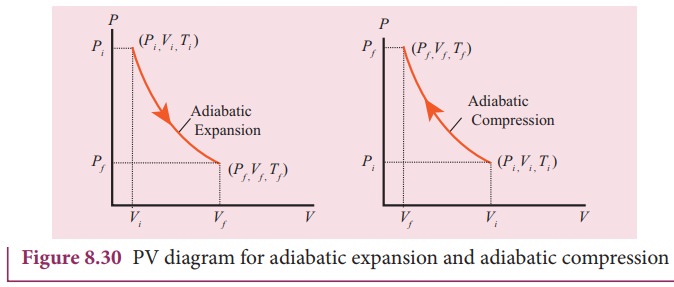
Adiabatic process - Thermodynamics

Which process is isothermal and which is adiabatic for this sealed system?

Why adiabatic PV curve is steeper than isothermal curve? Thermodynamics in Hindi
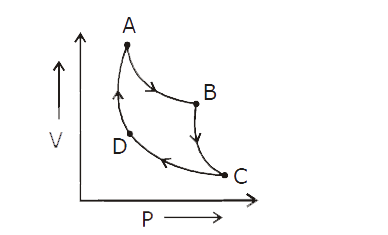
Show that the adiabatic curve is steeper than the isothermal curve.
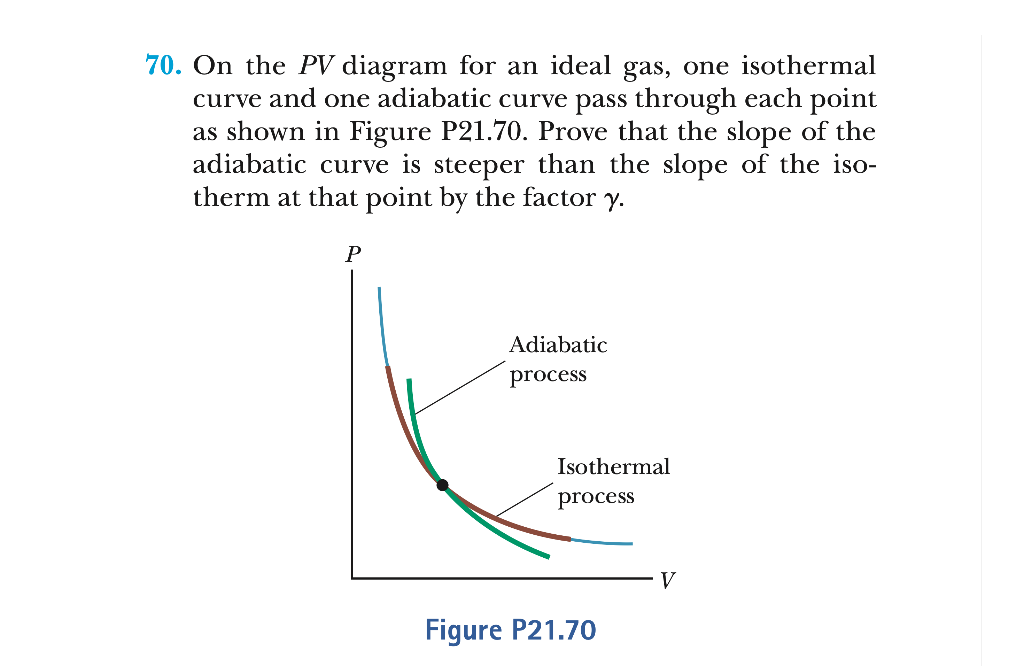
Solved 70. On the PV diagram for an ideal gas, one

Why is Adiabatic Curve steeper than Isothermal Curve
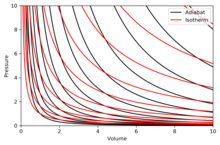
Adiabatic process - Wikipedia