ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Descrição

Safety and Efficacy of Upadacitinib in Patients With Active Ankylosing Spondylitis and an Inadequate Response to Nonsteroidal Antiinflammatory Drug Therapy: One‐Year Results of a Double‐Blind, Placebo‐Controlled Study and Open‐Label Extension - Deodhar

Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial. - Abstract - Europe PMC

The state of the art—psoriatic arthritis outcome assessment in clinical trials and daily practice - The Lancet Rheumatology
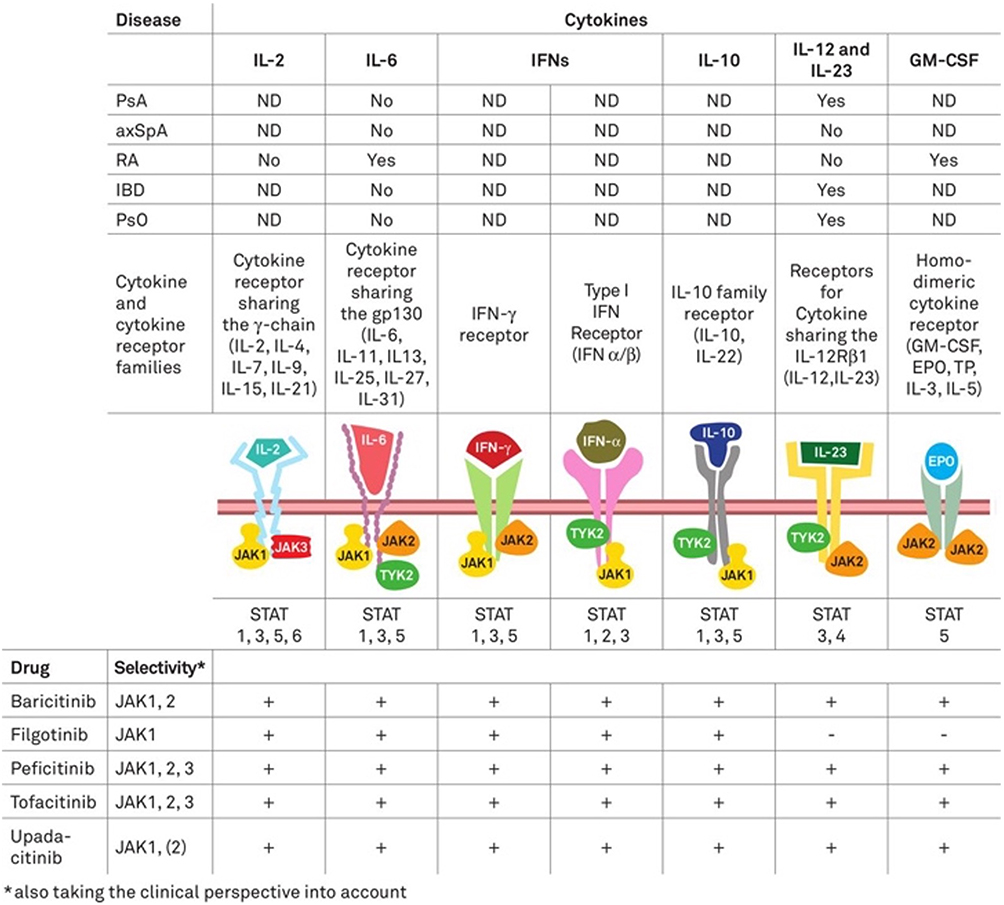
Management of axial spondyloarthritis

UCB Presents New Five-Year Data on BIMZELX® (bimekizumab-bkzx) in Ankylosing Spondylitis at ACR Convergence 2023

Table 6, Details of Included Studies - Upadacitinib (Rinvoq) - NCBI Bookshelf

Oral Abstracts - 2020 - International Journal of Rheumatic Diseases - Wiley Online Library

A and B, Percentages of patients in the golimumab (GLM) and placebo

Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. - Abstract - Europe PMC